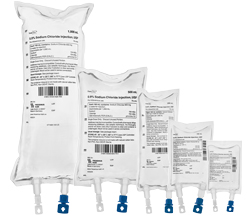
0.9% Sodium Chloride Injection, USP
(freeflex®)
| P.I. = Package Insert |
SDS
|
Product Information
- Latex Information
- Preservative Free
- Bar Coded
- Non-DEHP
- Non-PVC
- freeflex® is a registered trademark of Fresenius Kabi USA, LLC
Product Updates
- Unique ports are designed with finger guard. Ports are self-sealing after removing needle or spike.